When we talk about carbohydrates, we usually think of food rich in starch – rice, bread, and noodles. Or perhaps, you may have thought about food with a lot of sugar, such as sweets and dessert. Well, you are not wrong if you have thought about carbohydrates as such. Now that all this food talk made me hungry, let us discuss more deeply what carbohydrates are.
What are Carbohydrates?
Carbohydrates are biomolecules – this means that they are essential in making up a part of living things. Carbohydrates contain carbon and, as such, are considered organic compounds.
In science, carbohydrates are substances containing carbon, hydrogen, and oxygen. They usually have a general formula of Cx(H2O)y. Carbohydrates are comprised of three different categories – monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
Monosaccharides are monomers of carbohydrates – it is the simplest form of carbohydrates and cannot be further broken down. They have a general formula of (CH2O)x and show isomerism. Monosaccharide isomerism include aldose and ketose, open-chain and ring forms, as well as α-isomers and β-isomers. Monosaccharide isomerism includes aldose and ketose, open-chain and ring forms, as well as α-isomers and β-isomers.
All monosaccharides are either aldoses or ketoses, and this depends on the position of the carbonyl group of the monosaccharide. In hexose aldoses, such as glucose, the monosaccharide has a six-membered ring known as a pyranose ring. Conversely, in hexoses that are ketoses, such as fructose, the monosaccharide has a five-membered ring known as a furanose ring. While six-membered rings exist for fructose, the five-membered ring is more stable.
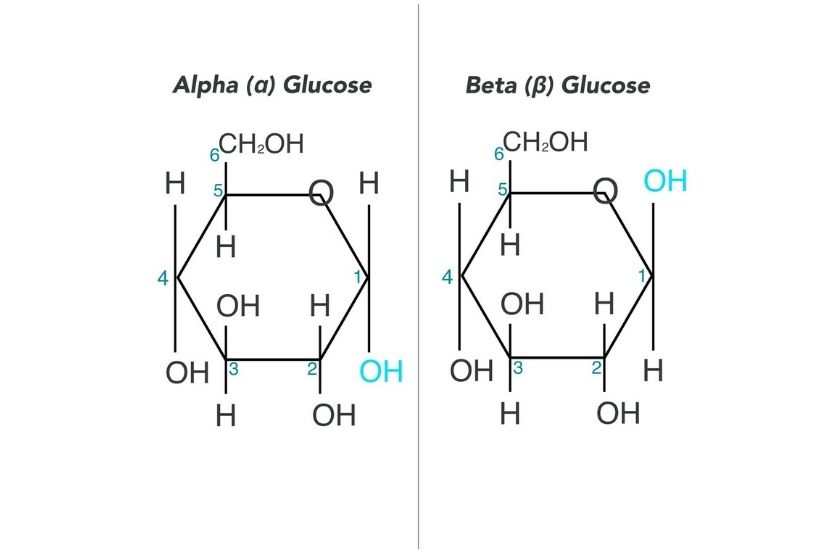
In the α-isomers and β-isomers of monosaccharides, the position of the hydroxyl (OH) group determines whether the molecule is an α-isomers or a β-isomers. If the OH group is below the carbon atom 1, it is an α-isomer. However, if the OH group is above the carbon atom 1, it is a β-isomer. Here is an example of α-isomers and β-isomers in glucose.
Disaccharides
When two monosaccharides are joined together via a condensation reaction, a disaccharide molecule would be formed. The monosaccharides are bonded with a glycosidic bond and can be separated via hydrolysis. Common disaccharides include maltose and lactose, which are reducing sugars, and sucrose, which is a non-reducing sugar.
Disaccharides are similar to monosaccharides in terms of their property – they are sweet, appear crystalline, and are readily soluble in water. This category of carbohydrates can be hydrolysed with acids or enzymes, splitting them back into their monomer form.
Maltose
Comprising of two glucose residues, maltose is formed when amylases digest starch. It is commonly found in animals and germinating seeds. This is seen in the process of brewing beer. Barley grains are used as carbohydrate sources in brewing. Yeast will ferment the maltose present into alcohol by converting maltose into glucose with the help of the enzyme maltase.
Lactose
Lactose is found only in milk and is a significant energy source for young mammals. It is made of glucose and galactose molecules, and it is digested slowly, which allows mammals to receive a steady release of energy.
Sucrose
The most abundant disaccharide in nature, sucrose, is most commonly found in plants and is used as cane sugar. It comprises glucose and fructose residues and is a good transport sugar due to its high solubility. That said, sucrose is the main component of sugars that you buy from stores for consumption.
Polysaccharides
Polysaccharides are carbohydrate macromolecules comprising hundreds, if not thousands of monosaccharides joined by glycosidic bonds. Being a macromolecule, polysaccharides are usually insoluble in water. Polysaccharides can be branched chains or unbranched chains. However, this depends on the isomerism of monosaccharides and the types of glycosidic bonds between monomers.
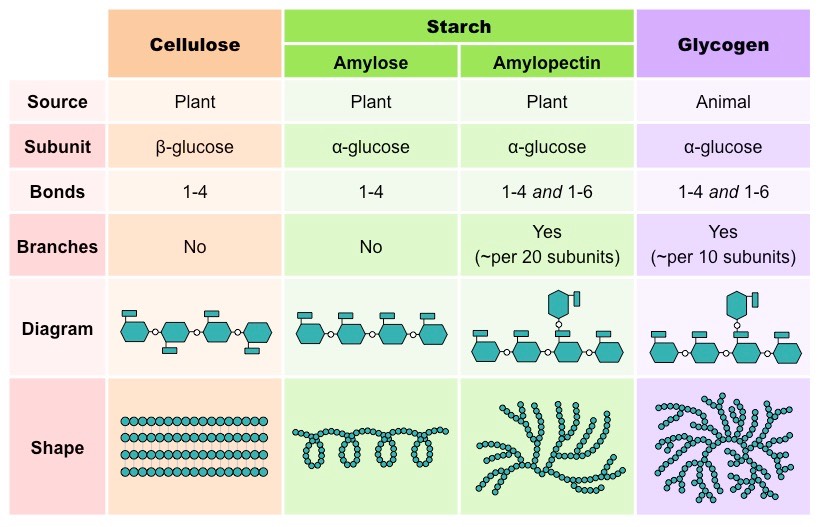
There are two general functions of polysaccharides – storage and structural. Some of the most common storage polysaccharides include starch and glycogen. Similarly, the most frequently seen structural polysaccharides include cellulose.
Starch
Found as small granules in most plant parts, starch is a polymer of α-glucose, and it is used to store energy from excess glucose produced during photosynthesis. Starch is a mixture of two types of carbohydrates – amylose and amylopectin. Starch comprises about 20% amylose and 79% amylopectin.
A relatively smaller molecule, amylose, comprises up to 300 glucose monomers, and it is an unbranched helical chain with α-1-4 glycosidic bonds between glucose residues. On the other hand, amylopectin is a much larger molecule comprising around 1,300 to 1,500 glucose monomers. Unlike amylose, it is a branched chain with both α-1-4 glycosidic bonds and α-1-6 glycosidic bonds between glucose residues.
So, what makes amylose a helical chain? Well, the α-1-4 glycosidic bonds have a bond angle of about 109°. This causes the polymer to be twisted into a helix, giving amylose its unbranched helical shape. That said, you might be curious – what about the α-1-6 glycosidic bonds in amylopectin? For every α-1-6 glycosidic bond formed, a branched point is inserted in the amylopectin polymer. This occurs every 24 to 30 glucose residues.
With such bonding and structures, both amylose and amylopectin are highly compact polysaccharides. These two parts will entangle to form a complex 3D structure – starch. As such, starch is a good form of storage for plants.
Glycogen
Unlike starch, glycogen is found in animals and fungi. It serves as the significant polysaccharide storage located primarily in the liver and muscles. As these two organs have high metabolic activity, glycogen provides a good energy reserve. However, similar to starch, it is comprised of α-glucose residues and exists as granules.
Structurally, glycogen appears to be similar to amylopectin. Glycogen is made up of α-glucose residues joined together by both α-1-4 glycosidic bonds and α-1-6 glycosidic bonds. However, it contains fewer glucose residues and has shorter chains of 10 to 20 glucose residues each. Glycogen is also more highly branched than amylopectin.
Now, how do the structures and properties of starch and glycogen result in their storage function?
Since both are large molecules, starch and glycogen are insoluble in water, which barely affects the cell’s water potential. A large number of α-glucose monomers also mean that there are many carbon atoms, which are building blocks of the cell. The α-1-4 glycosidic bonds result in both starch and glycogen being helical coils, and this causes them to store many glucose residues in a small volume. This makes both starch and glycogen suitable for storage.
Cellulose
We have been discussing a lot about storage carbohydrates. That said, let us talk about structural carbohydrates. Cellulose is a structural component of all plant cells, making up 20% to 40% of plant cell walls, and this makes it the most widely found organic molecule on our planet.
Cellulose is a polymer of β-glucose, with about 10,000 glucose monomers per chain, unlike the storage carbohydrates. The β-glucose monomers are joined together by β-1-4 glycosidic bonds. As such, every alternate residue must be rotated 180°. The β-1-4 glycosidic bonds also make cellulose chains straight.
OH, groups from glucose residues are projected outwards, forming hydrogen bonds with glucose residues from neighbouring chains. This results in cross-linking, which binds cellulose chains tightly together. Micelles are formed when bundles of about 40 chains are aggregated. They are then arranged to form microfibrils, which combine to form macrofibrils.
Being a large molecule, cellulose is insoluble in water, making it ideal as a structural carbohydrate as it remains intact in the presence of water. On top of that, cellulose is straight-chained due to β-1-4 glycosidic bonds, which makes hydrolysing the bonds difficult, thus cellulose is stable. Inter-chain bonding binds cellulose chains tightly together, giving rise to its excellent tensile strength.
Conclusion
Now that we know that carbohydrates are not just about food, we can also appreciate their importance to life on earth. Carbohydrates are not just essential in humans but to plants and animals as well. That said, do remember about carbohydrates the next time you put food into your mouth!





Hey, can you do posts on theorems in math? Like, for example, the Cayley Hamilton Theorem, Bayes Theorem, and the Apolloniu’s Theorem to start with? Of course, it’s just a suggestion, but I like your website and I would prefer to learn it from your own words (the topics are what we’re revising in my class, btw). Thanks man,
Jake
Hi, appreciate your comment and suggestions! I’m actually not very good at math, but I can definitely try writing a couple of mathematical concepts that I am more confident in. This isn’t actually my website as well, I’m just a writer here 🙂 All the best in your studies!