Unless you have studied chemistry at some point in your life, you probably would not know what alkenes are. However, if I were to tell you that alkenes are used to make plastic, you would probably have a better idea. Alkenes also have properties that are similar to alkanes, which makes them suitable fuels as well. Today, we shall discuss what alkenes are and some of the reactions involving alkenes.
What are Alkenes?
As mentioned in Organic Chemistry – An Introduction, alkenes are hydrocarbons with a general formula of CnH2n. To be more specific, alkenes are saturated aliphatic hydrocarbons, which means that they only contain carbon and hydrogen atoms. This makes alkenes similar to alkanes. Covalent bonds join adjacent carbon atoms. We have also mentioned that alkenes have a suffix –ene.
Here are some of the simpler alkanes that we commonly encounter.
| n | Name | Molecular formula | Structural formula | Mr | Melting point / °C | Boiling point / °C | Physical state (r.t.p) |
| 2 | Ethene | C2H4 | CH2=CH2 | 28 | -169 | -104 | Gas |
| 3 | Propene | C3H6 | CH3CH=CH2 | 42 | -185 | -48 | Gas |
| 4 | Butene | C4H8 | CH3CH2CH=CH2 | 56 | -185 | -6 | Gas |
| 5 | Pentene | C5H10 | CH3(CH2)2CH=CH2 | 70 | -165 | 30 | Liquid |
| 6 | Hexene | C6H12 | CH3(CH2)3CH=CH2 | 84 | -140 | 63 | Liquid |
“Methene” does not exist since it only has one carbon atom. As such, there is no double bonds between carbon atoms to form an alkene.
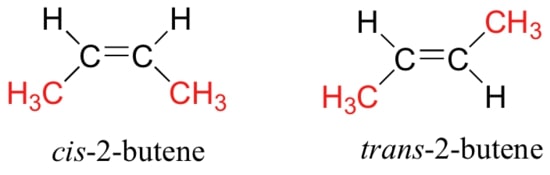
Alkenes with three or more carbon atoms exhibit isomerism. This includes constitutional isomerism due to the arrangement of carbon atoms, the position of C=C bond, and cis-trans isomerism due to the restricted rotation of atoms about the C=C bond.
For example, butene has four isomers – but-1-ene (CH3CH2CH=CH2), 2-methylpropene (CH2=C(CH3)2), cis-but-2-ene, and trans-but-2-ene.
What are Cycloalkenes?
Cycloalkenes are saturated alicyclic hydrocarbons – this means that they have a ring of carbon atoms. Unlike alkenes, cycloalkenes have a general formula of CnH2n-2. They are technically different from alkenes and contain the prefix cyclo– to distinguish them from the aliphatic alkenes. This makes cycloalkenes similar to cycloalkanes.
Here are some of the simpler cycloalkanes that we often see.
| n | Name | Molecular formula | Structural formula | Mr | Melting point / °C | Boiling point / °C | Physical state (r.t.p) |
| 3 | Cyclopropene | C3H4 | 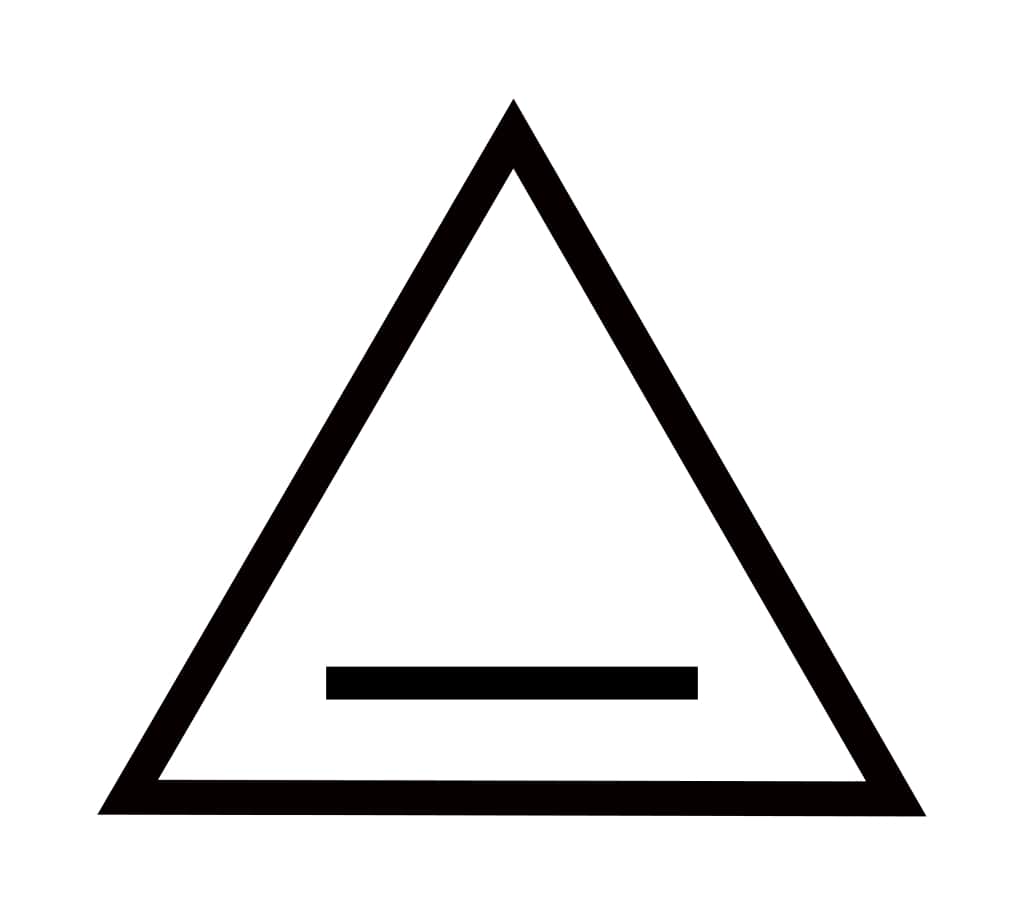 | 40 | -127 | -33 | Gas |
| 4 | Cyclobutene | C4H6 | 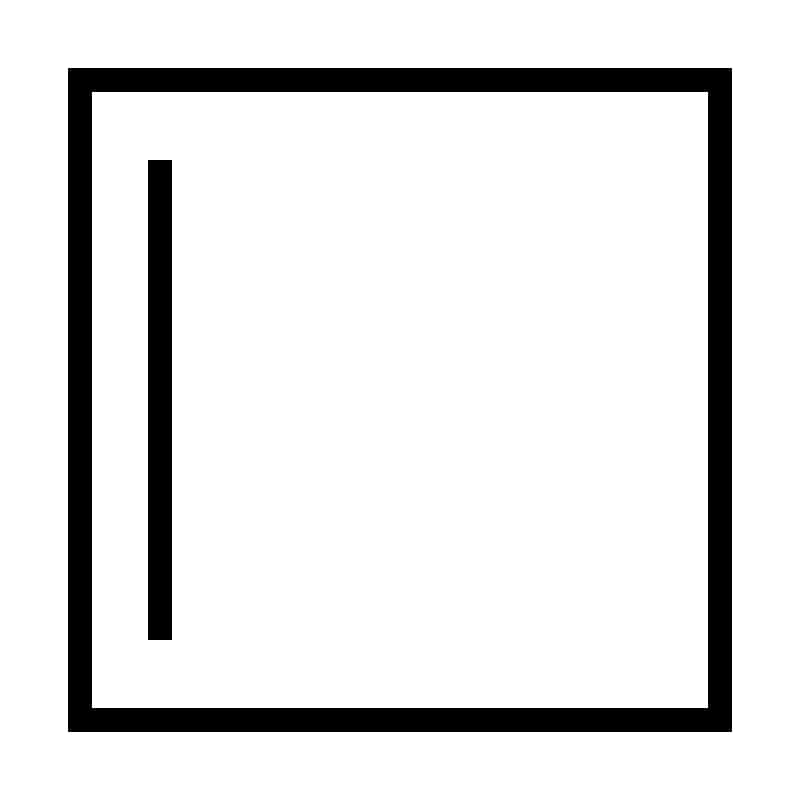 | 54 | -90 | 13 | Gas |
| 5 | Cyclopentene | C5H8 | 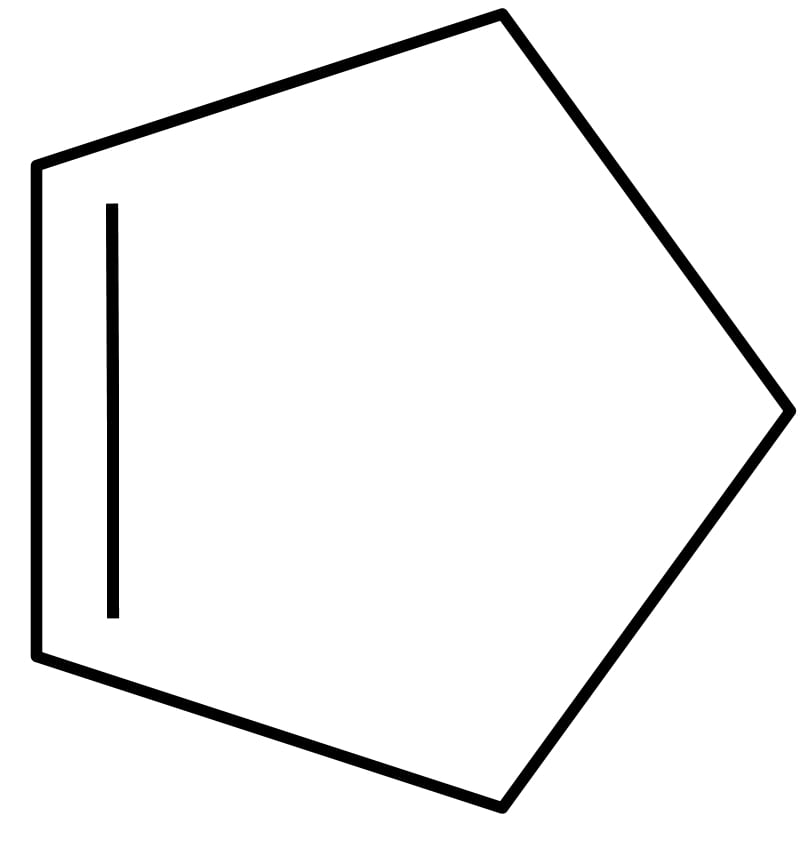 | 68 | -94 | 49 | Liquid |
| 6 | Cyclohexene | C6H10 | 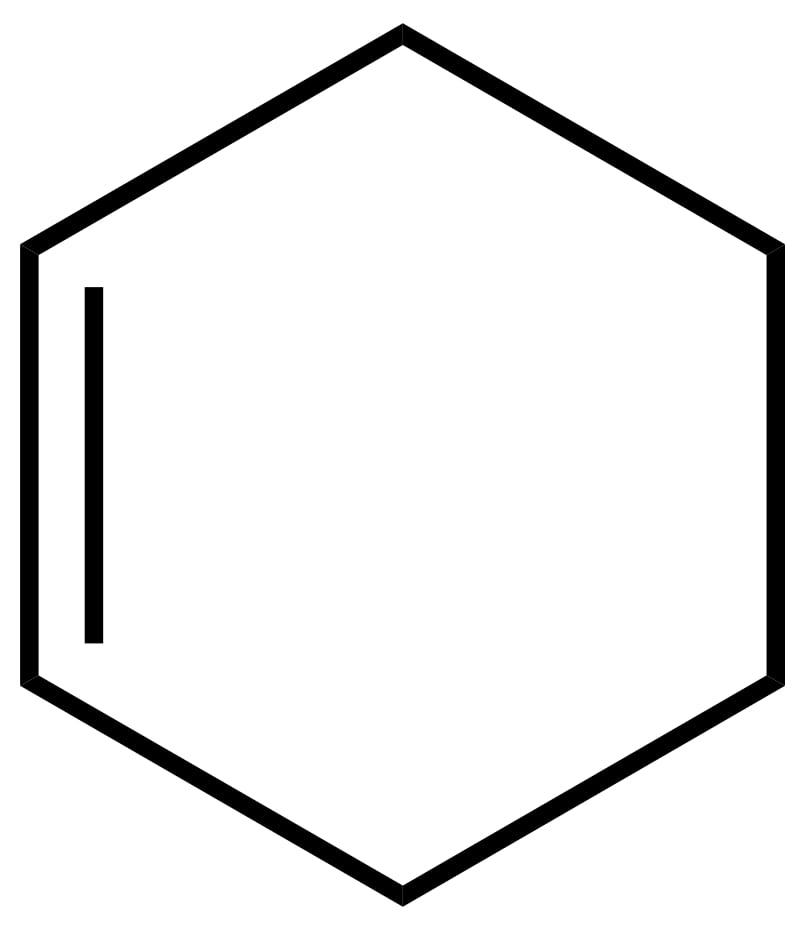 | 82 | 7 | 81 | Liquid |
Properties of Alkenes
As mentioned earlier, alkenes have similar physical properties as alkanes.
Melting and boiling points
As the number of carbons in the hydrocarbon chain increases, more energy is needed to overcome the stronger instantaneous dipole-induced dipole (id-id) interactions between molecules. As such, the boiling point of the straight-chained alkane isomers increases. Therefore, the first three alkenes are gases at room temperature, whereas subsequent alkenes are liquids then solids. Likewise, this is similar to alkanes.
Branched-chained alkene isomers often have lower melting points than straight-chained alkene isomers. This is because they have less surface area of contact between molecules since they are more spherical. As such, there is a decrease in the strength of id-id interactions.
Cis alkene isomers are polar molecules as they have a larger net dipole moment than their non-polar trans counterparts. As such, there is strong permanent dipole-permanent dipole (pd-pd) interactions between molecules. This gives rise to higher boiling points as more energy is needed to break the stronger pd-pd interactions. However, trans alkene isomers have higher melting points as they are more closely packed.
Other physical properties
Alkenes are colourless, non-polar molecules. They are insoluble in polar solvents but are soluble in non-polar solvents. This is due to the energy released between water molecules and alkene molecules being insufficient to overcome the strong hydrogen bonds between water molecules.
On top of that, alkenes are less dense than water. They also have stronger smells as compared to alkanes.
Preparing Alkenes
There are three ways to prepare alkenes – catalytic cracking of petroleum, dehydration of alcohols, and dehydrohalogenating halogenoalkanes. While catalytic cracking will produce a mixture of alkanes and alkenes, the other methods will only produce alkenes.
Catalytic Cracking of Petroleum
Catalytic cracking involves breaking down hydrocarbon chains into smaller alkanes and alkenes with a catalyst at a temperature of 600°C. The catalyst used is usually aluminium oxide or silicon (IV) oxide.
Cracking is used to produce petroleum fractions in demand, such as petrol and naphtha. On top of that, it can also produce hydrogen, which is needed in the Haber process to produce ammonia.
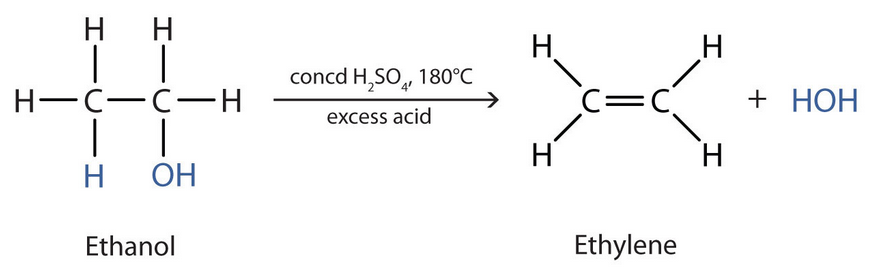
Dehydration of Alcohol
We can dehydrate alcohols in an elimination reaction under heat. However, excess concentrated sulfuric acid (H2SO4) or aluminium oxide (Al2O3) is needed.
Dehydrohalogenating Halogenoalkanes
Similar to the dehydration of alcohols, we can dehydrohalogenate halogenoalkanes in an elimination reaction under heat. In this case, either potassium hydroxide or sodium hydroxide in ethanol is used as a reactant. Two products will be formed – the major product (more substituted alkene) and the minor product (less substituted alkene). The major product has more alkyl groups bonded to the carbon atoms bound by the C=C bond.
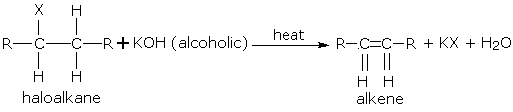
Reactions
Electrophilic Addition
Pi electrons in the C=C bond are more exposed than sigma electrons, making them a good source of electrons. This causes the C=C bond to have a high electron density, making the alkene more susceptible to electrophile attacks. As such, alkenes are more reactive than alkanes.
That said, alkenes generally undergo electrophilic addition. This occurs when the weak pi bond in the C=C bond is broken to form two strong sigma bonds.
| Reaction | Reagents and Conditions | Mechanism |
| Adding hydrogen halides | Gaseous hydrogen halides | Symmetrical alkenes 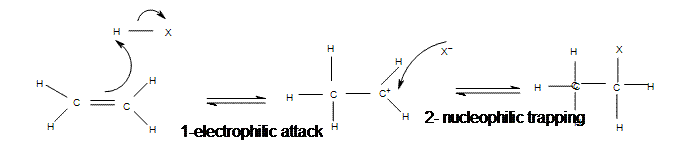 Asymmetrical alkenes 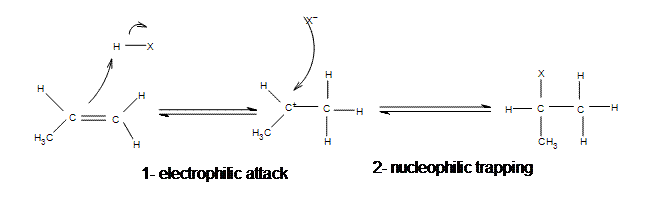 |
| Adding halogens in CCl4 | Halogens in CCl4 as solvent | 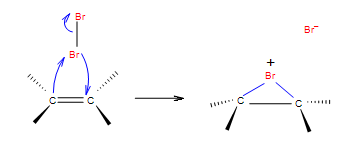 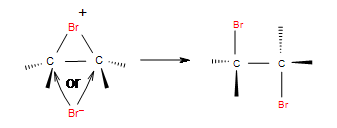 |
| Adding halogens in water | Halogens in water or aqueous halogens | 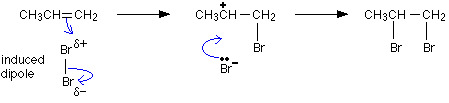 |
| Adding water | Cold concentrated H2SO4, heat with water OR steam, heat, high pressure, H3PO4 catalyst | 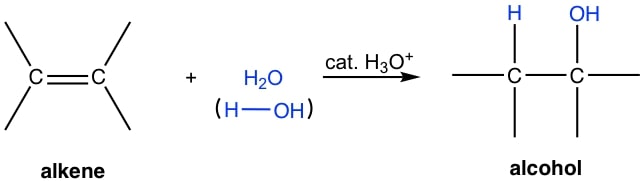 |
Reduction
The reduction of alkenes form alkanes. For more information, read our article on alkanes.
Oxidation
| Reaction | Reagents and Conditions | Mechanism |
| Combustion | Oxygen | In limited oxygen, alkenes may burn to give carbon monoxide and water. E.g. C2H4 (g) + 2O2 (g) → 2CO (g) + 2H2O (l) In excess oxygen, alkenes burn to give carbon dioxide and water. E.g. C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (l) This is similar to the combustion of alkanes. However, alkenes burn with a more smoky flame due to a higher concentration of carbon atoms. |
| Diol formation | Cold KMnO4 in aqueous NaOH | The C=C bond undergoes mild oxidation when there is no heating. This results in the breaking of the pi bond and forming of the diol. |
| Carboxylic acid, ketones and CO2 formation | KMnO4 in aqueous H2SO4, heat | The C=C bond undergoes strong oxidation when there is heat. This results in the cleaving of the pi bond, and then the cleaving of the sigma bond. |



Add comment