Chemical bonding plays an important role in Chemistry – they help to form compounds with elements. Today, we will be discussing the different types of bonds and intermolecular forces of attraction.
Covalent Bonding
Covalent bonds are strong electrostatic forces of attraction between a shared pair of electrons and the nuclei of each atom involved in the bonding. They are usually formed between two atoms with small differences in their electronegativities. This occurs when two atomic orbitals are overlapped to share electron pairs between two atoms. There are two types of covalent bonds – sigma bonds and pi bonds.
Sigma bonds are the head-on overlap of orbitals. Each covalent bond contains only one sigma bond. On the other hand, pi bonds are the sideway overlap of orbitals that occur when there is already a sigma bond. Pi bonds are weaker compared to sigma bonds as there is a smaller degree of overlap between orbitals.
There are simple covalent molecules and giant covalent solids.
Properties
Little energy is needed to break the weak intermolecular forces of attraction between molecules. As such, covalent molecules have low melting points. We will discuss intermolecular forces of attraction later.
Without free-moving particles or delocalised electrons, covalent compounds cannot conduct electricity in any state. However, this does not apply to compounds that form ions when reacted with water. That said, most covalent compounds are insoluble in water, but can dissolve in polar solvents.
Factors
The strength of covalent bonds is determined by their bond energy. Two factors affect the bond energy of covalent bonds – atomic radius and the number of bonds.
The bigger the atomic radius, the smaller the extent of orbital overlap, thus the covalent bond is less effective. On the other hand, the greater the number of bonds, the stronger the covalent bond.
Dative Covalent Bonds
Dative covalent bonds are covalent bonds formed from electrons shared by only one of the atoms. The donor atom must have a lone pair of electrons whereas the recipient atom must have vacant orbitals in its valence electron shell.
Ionic Bonding
Ionic bonds refer to the strong electrostatic forces of attraction between ions of opposite charges. They are formed when one or more electrons are transferred between atoms, forming a cation and an anion with noble gas configurations. Ionic bonds are often formed between two elements that have a large electronegative difference – usually metals and non-metals.
Ionic compounds have a giant ionic lattice structure. In the giant ionic lattice, cations attract anions and anions attract cations. As such, ionic bonds are non-directional and is equally strong from every direction.
Properties
Ionic compounds have strong electrostatic forces of attraction between ions of opposite charges. As such, a lot of energy is needed to break ionic bonds. This gives rise to the high melting and boiling points of ionic compounds. We can estimate the magnitude of melting and boiling points of ionic compounds using their lattice energy.
Ions in solid ionic compounds have a fixed position and are unable to conduct electricity. However, ions in aqueous and molten ionic compounds are delocalised and can move around freely to conduct electricity. This means that ionic compounds can conduct electricity in aqueous and molten states.
Ions in ionic compounds have a regular lattice pattern, which makes them hard and brittle.
Factors
We can estimate ionic bond strength by the lattice energy. Lattice energy is the amount of energy released when one mole of the ionic compound is formed from its constituent gaseous ions.
The magnitude of lattice energy ∝ (cation charge)(anion charge) ÷ (cation radius + anion radius).
As such, ionic bonds with greater ionic charges and smaller interionic distances are stronger.
Metallic Bonding
Metallic bonding is the electrostatic force of attraction between a lattice of positive metal ions and a sea of delocalised electrons. Similar to ionic compounds, metals have a giant metallic lattice structure. Metallic bonds are non-directional and are equally strong from every direction.

Properties
Metals have moderately high melting points since we do not need to break the attraction between the cations and electrons. However, metals have a high boiling point since we must completely separate all electrons from the cations.
Unlike ionic bonds, metals are good conductors of electricity in both solid and molten states. This is due to the ability of delocalised electrons to serve as charge carriers when we apply a potential difference.
Metals are also ductile and malleable since atom layers can slide over each other.
Factors
There are two main factors affecting the strength of metallic bonds – charge density and the number of delocalised electrons.
Charge density = charge of ion ÷ size of ion.
As such, there are stronger metallic bonds if there is a high charge density of cations since there are stronger electrostatic forces of attraction between cations and delocalised electrons.
With more delocalised electrons, there is more attraction between cations and delocalised electrons. This makes metallic bonds stronger.
Intermediate Bonding
Rather than being absolutely covalent or ionic, the bonds of most compounds are found on the bonding continuum. They are between ionic and covalent based on the electronegativity difference between compounds.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
As covalent bonds are directional, we use the VSEPR to predict the shape of covalent molecules. There are three main assumptions:
- Electron pairs tend to be as far as possible, minimising electronic repulsion to ensure the stability of the molecule. Both lone pair (lp) and bond pair (bp) electrons are considered electron pairs.
- Repulsions have the order lp-lp > lp-bp > bp-bp.
Shapes of Molecules
| Electron pairs | Lone pairs | Molecular geometry |
| 2 | 0 | Linear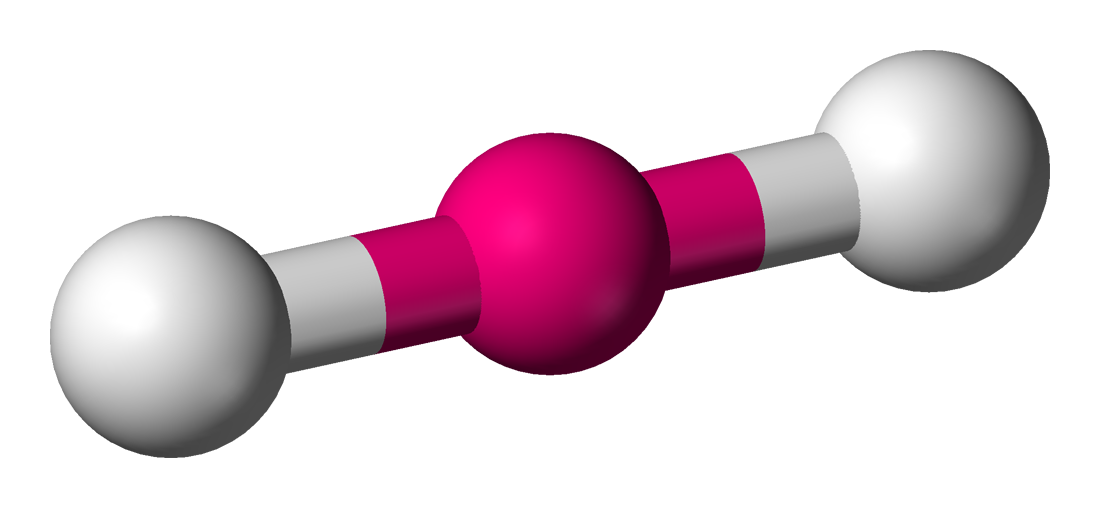 |
| 3 | 0 | Trigonal Planar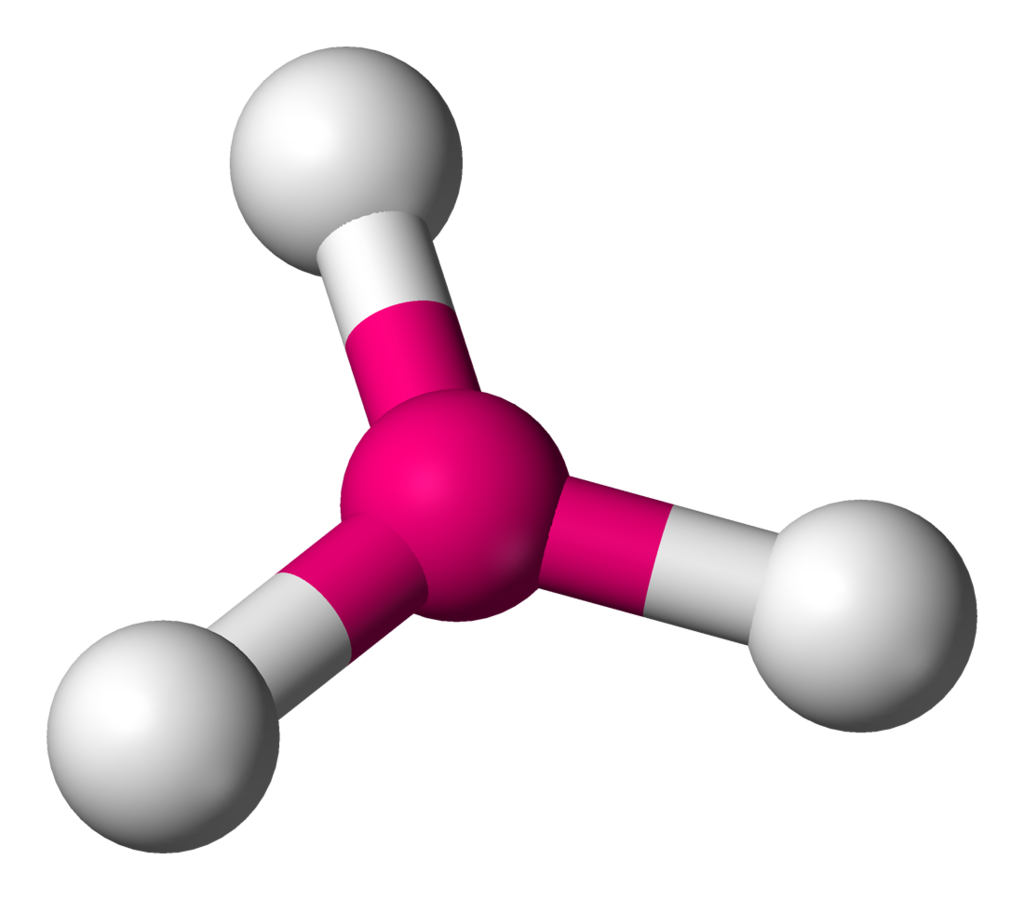 |
| 3 | 1 | Bent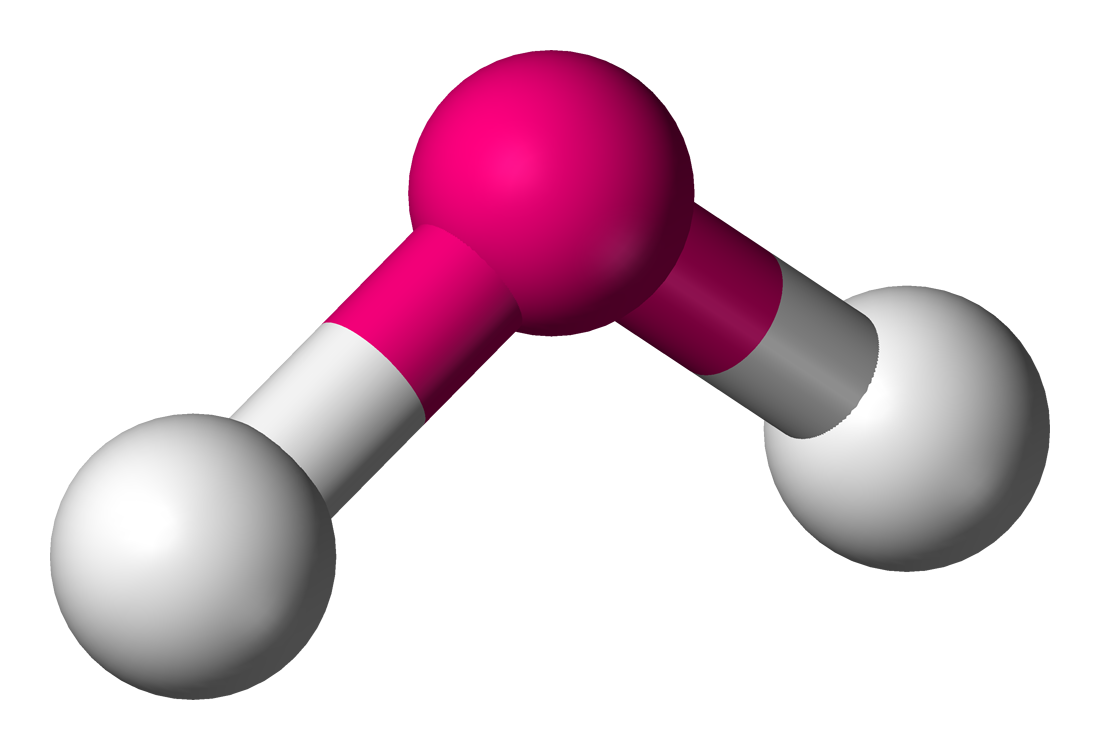 |
| 4 | 0 | Tetrahedral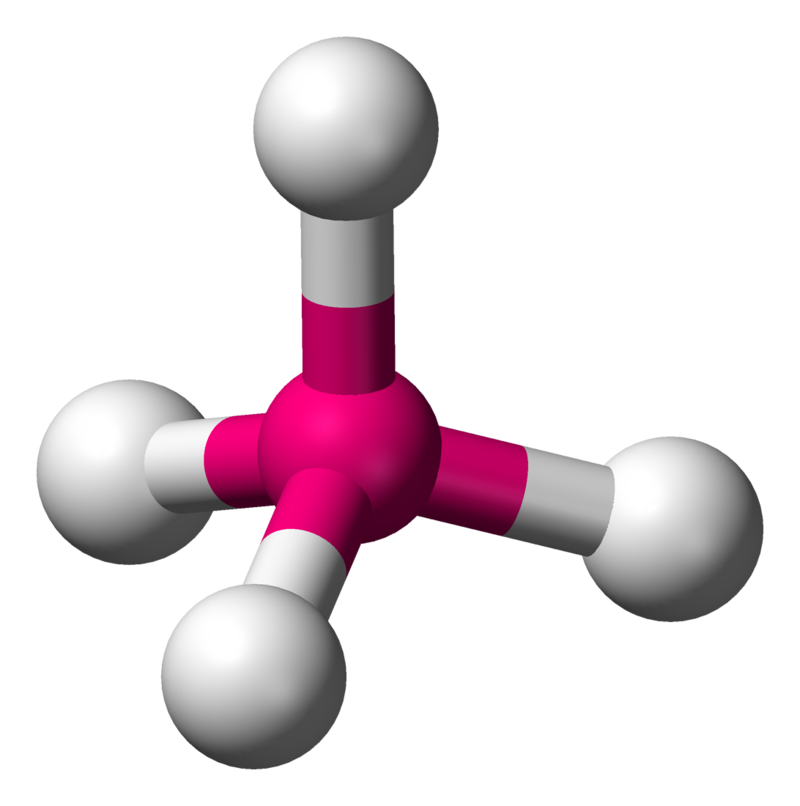 |
| 4 | 1 | Trigonal Pyramidal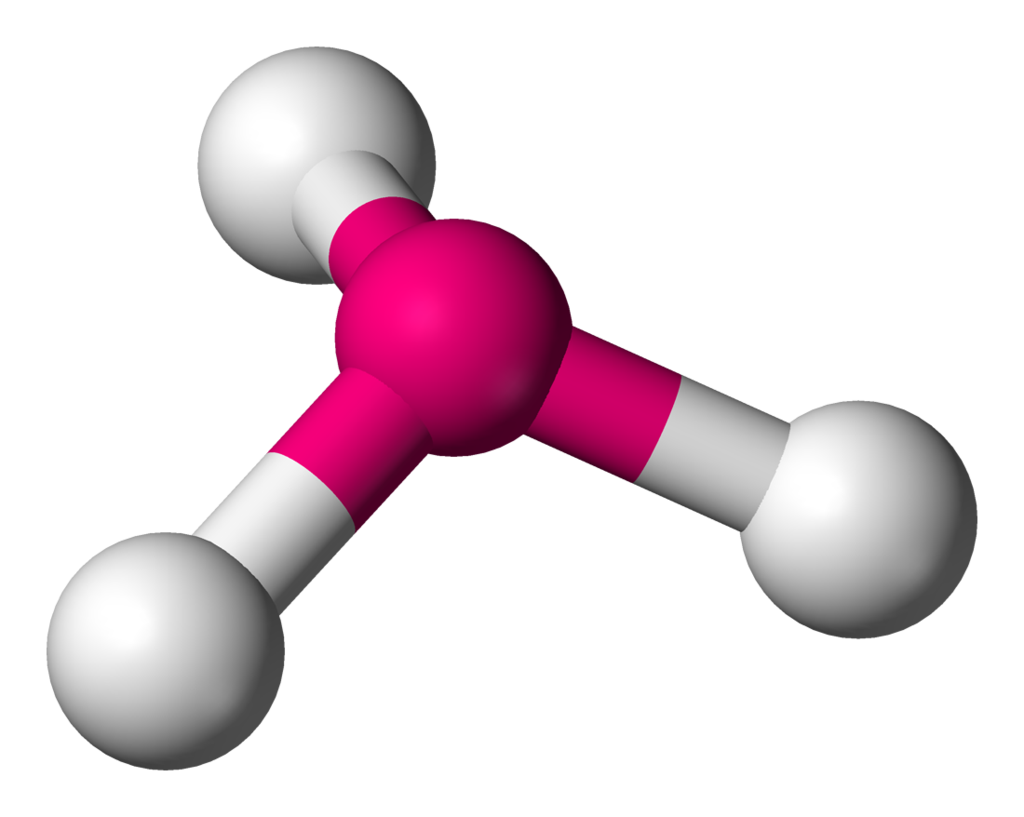 |
| 4 | 2 | Bent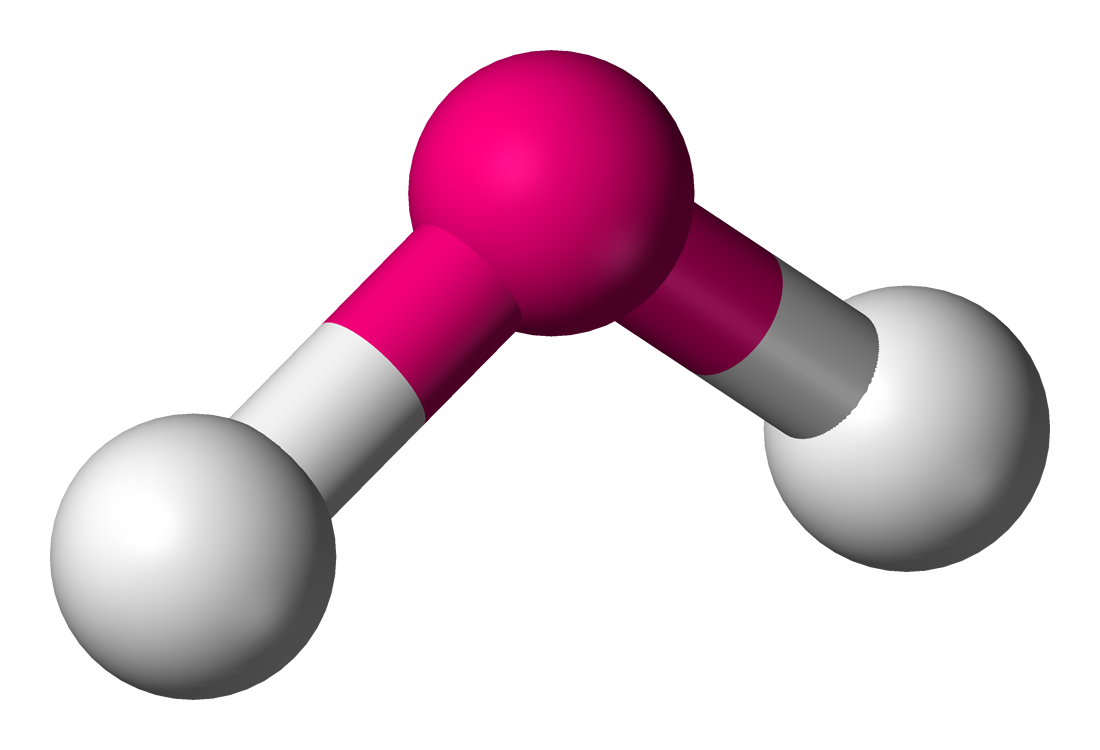 |
| 5 | 0 | Trigonal Bipyramidal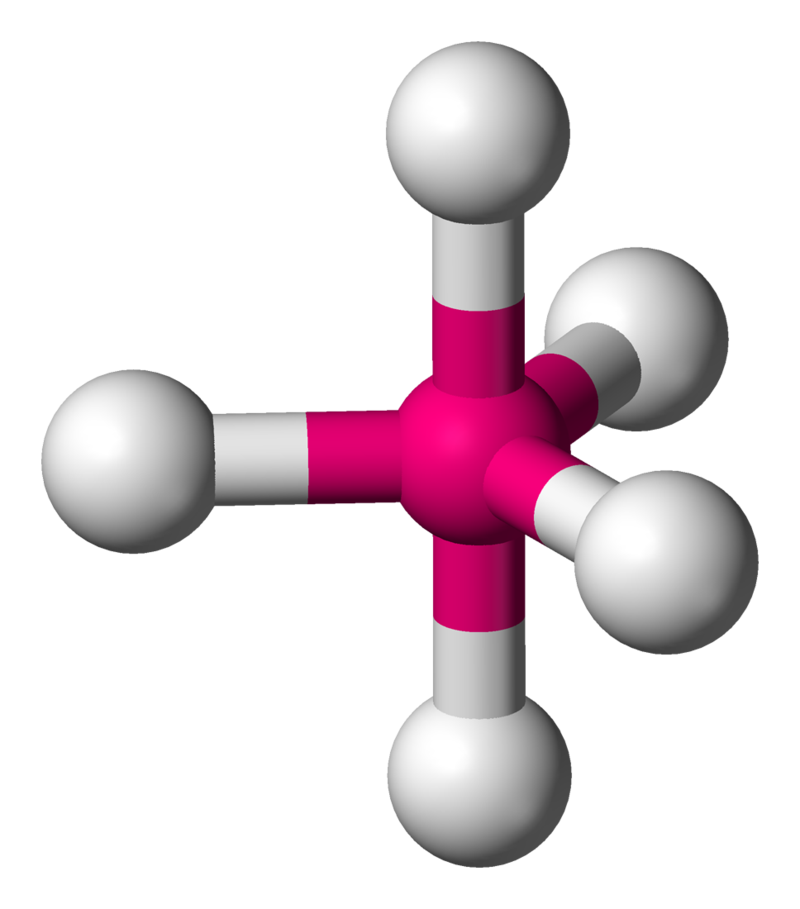 |
| 5 | 1 | Distorted Tetrahedral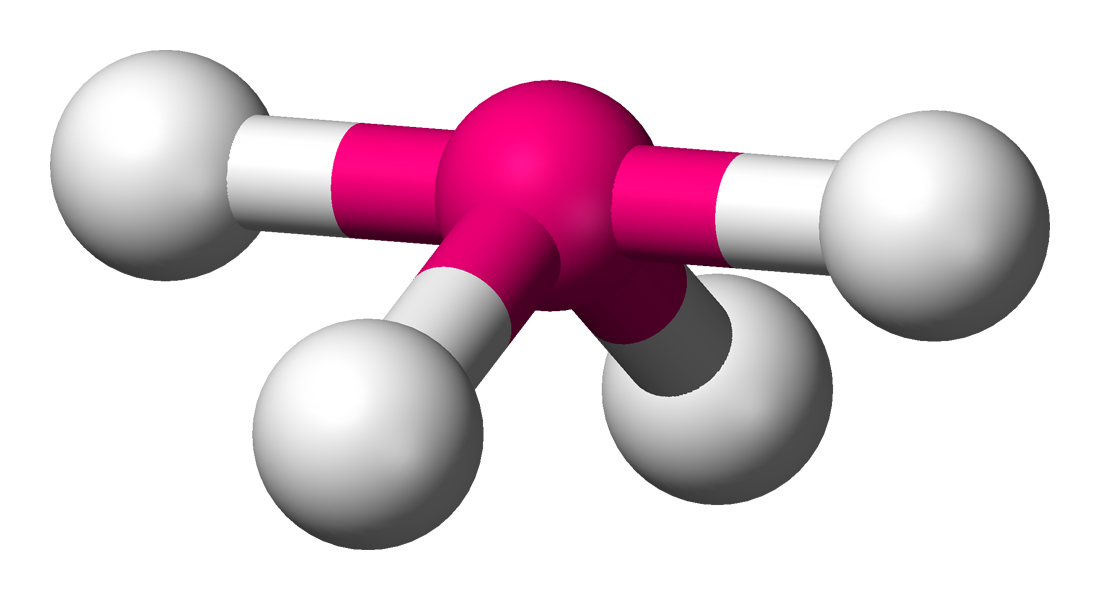 |
| 5 | 2 | T-shape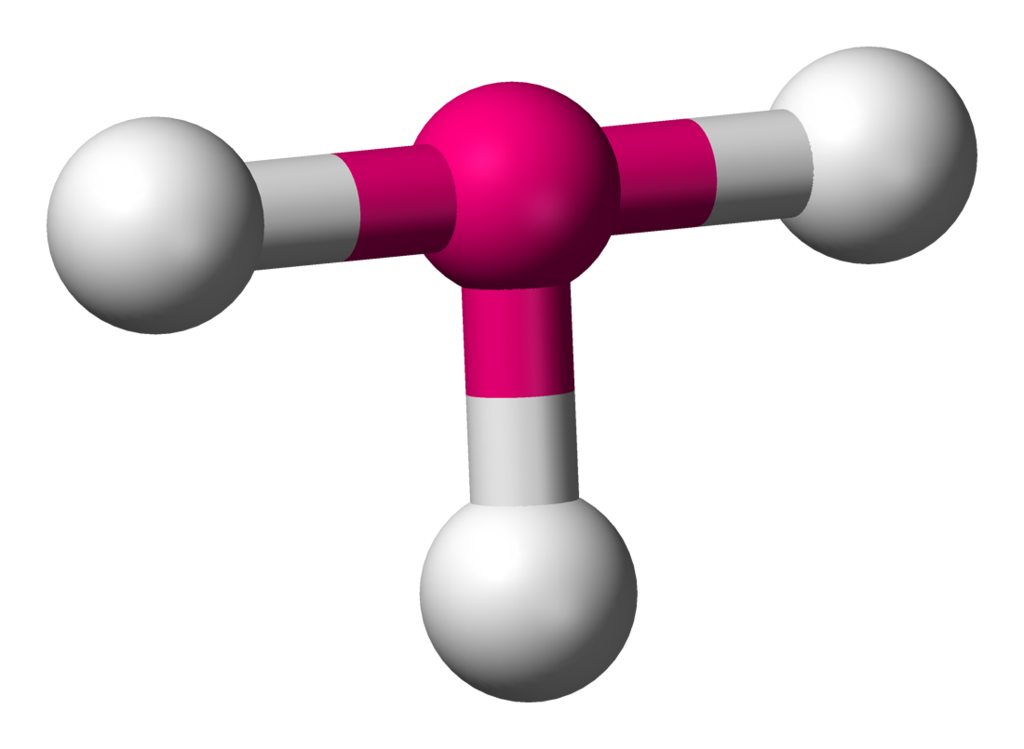 |
| 5 | 3 | Linear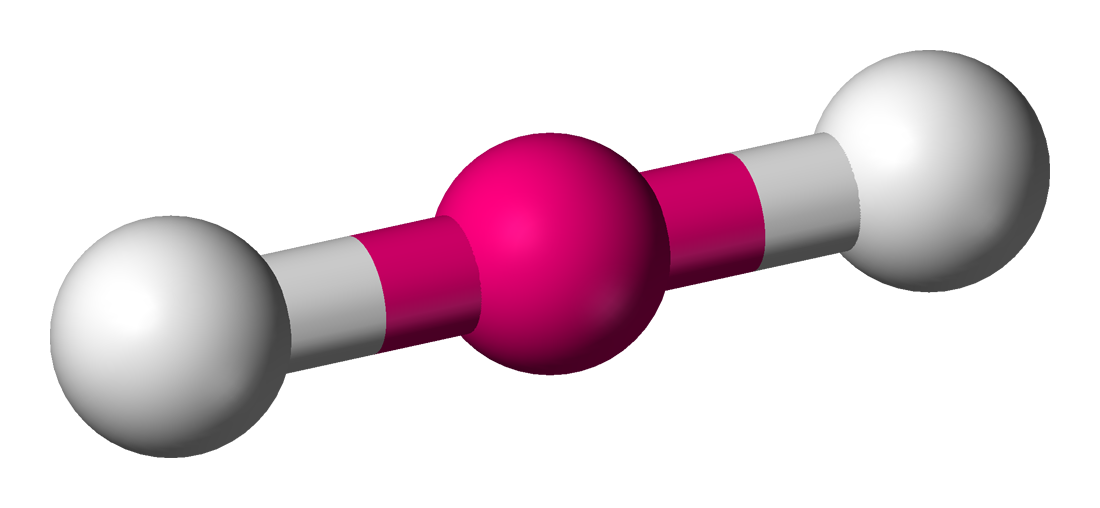 |
| 6 | 0 | Octahedral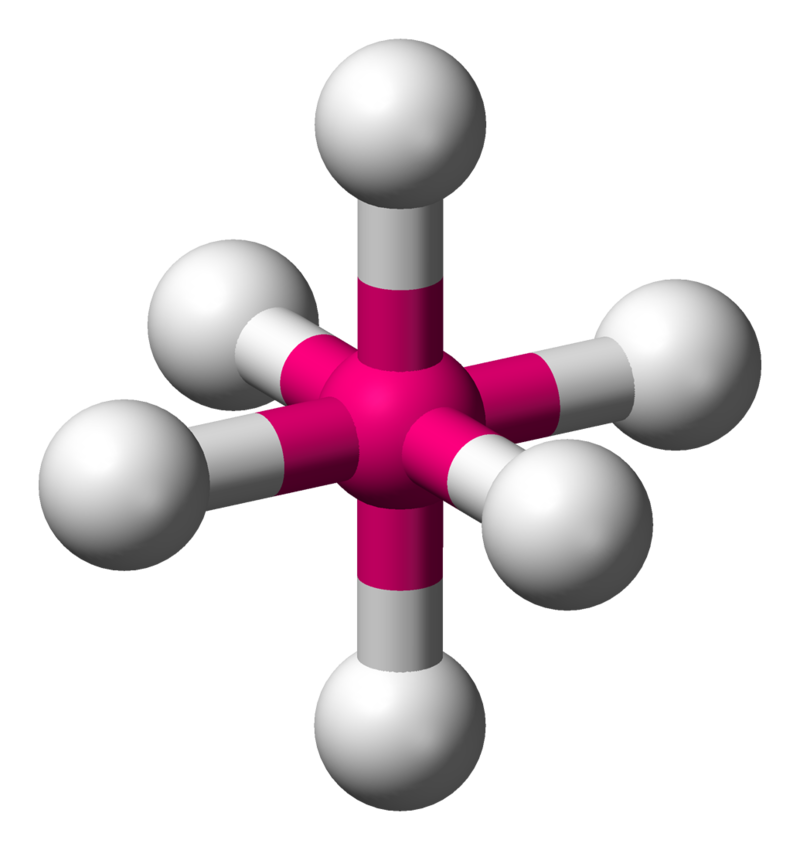 |
| 6 | 1 | Square Pyramidal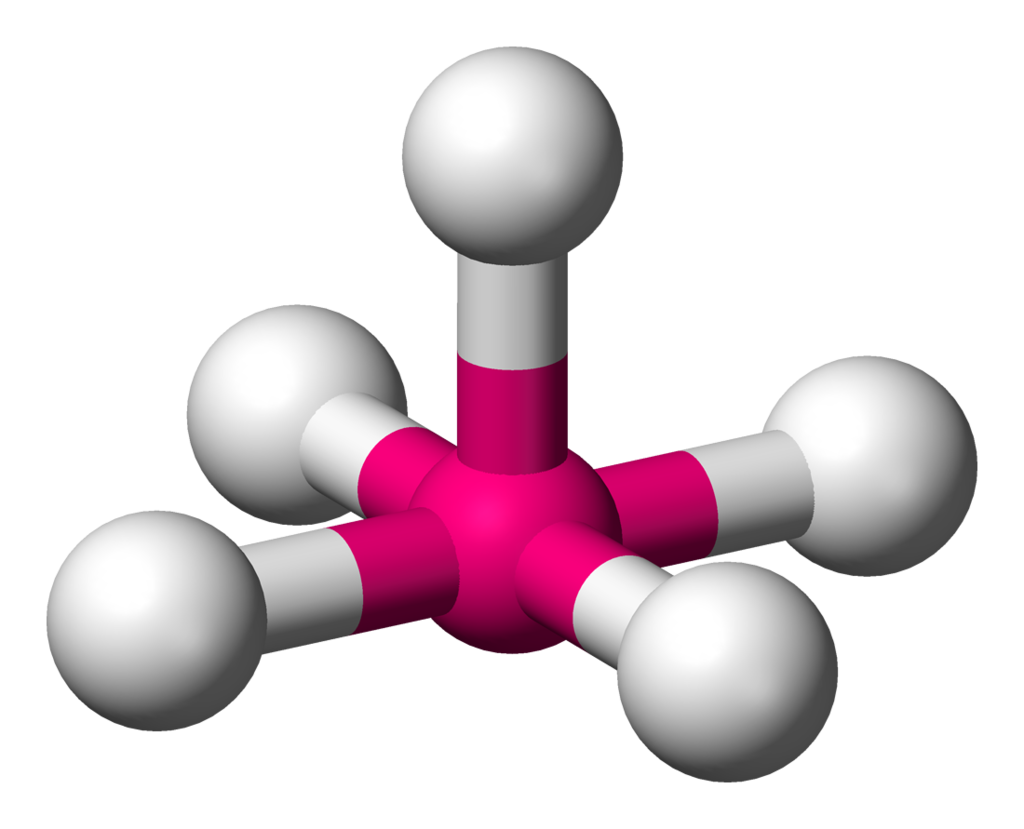 |
| 6 | 2 | Square Planar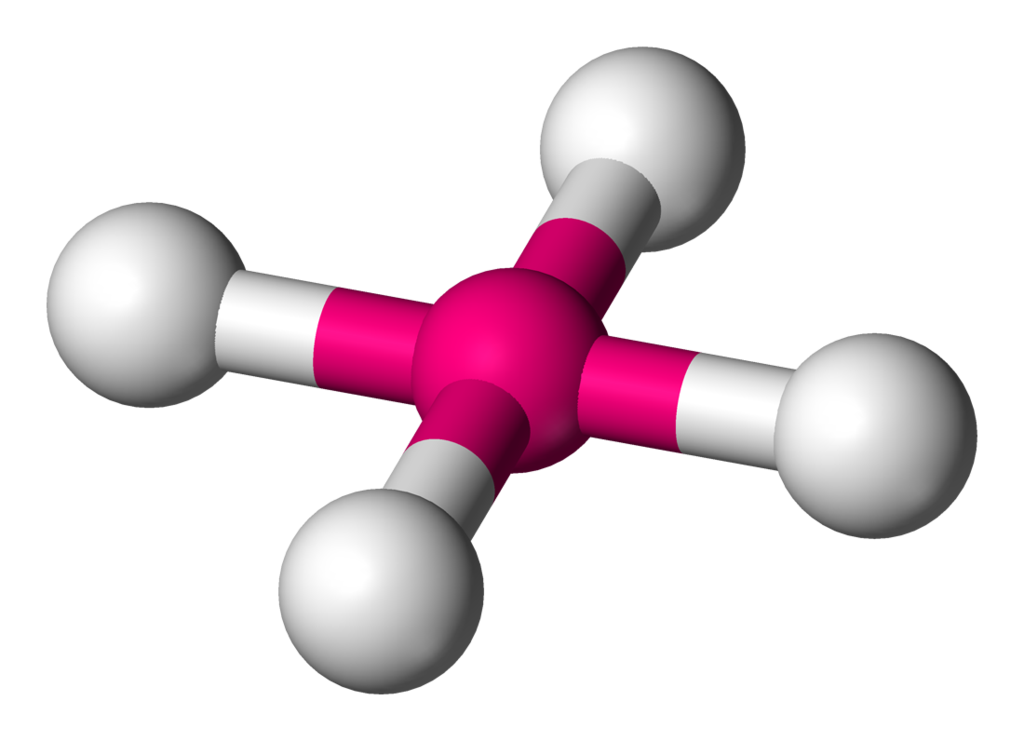 |
There are more shapes beyond six electron pairs. However, those are relatively uncommon. Hybrid orbitals are also formed when hybridisation occurs.
Polarity
When two atoms of equal electronegativity are bonded by a covalent bond, they are considered non-polar since the two bonded atoms share electrons equally. However, when two atoms of different electronegativities are bonded, they are polar as they share electrons unevenly. Polarity is a vector quantity with a direction and magnitude. The dipole moment measures the magnitude of polarity.
If the overall dipole moment of a molecule is not zero, the molecule is considered to be polar. Polar molecules have a partially positive (δ+) and partially negative (δ-) region.
Intermolecular Forces
The strength of intermolecular forces is as such for molecules with a similar number of electrons:
Hydrogen bonding > pd-pd interactions > id-id interactions
Instantaneous Dipole-Induced Dipole Interactions
Instantaneous dipole-induced dipole (id-id) interactions are intermolecular forces of attraction significant in non-polar molecules. However, they are found in all molecules.
The strength of id-id interactions depends on the number of electrons and the surface area. The more electrons, the more widespread the orbitals, which makes polarising of orbitals easier. As such, id-id interactions form more easily and are stronger. On the other hand, straight-chain molecules have a larger surface area than branched molecules, thus more electrons interact to form stronger id-id interactions.
Permanent Dipole-Permanent Dipole Interactions
Permanent dipole-permanent dipole (pd-pd) interactions are intermolecular forces of attraction between poles of opposite charges in polar molecules. They form when the δ+ end of one molecule attracts the δ- end of another molecule, together with id-id interactions.
If molecules have a similar number of electrons, those with pd-pd interactions tend to have higher boiling points than those with id-id interactions. That said, molecules with greater polarities will have stronger pd-pd bonds and thus higher boiling points.
Hydrogen Bonding
Hydrogen bonds are weak electrostatic forces of attraction. They are found between a hydrogen atom that is covalently bonded to an atom with strong electronegativity (often fluorine, oxygen, or nitrogen) and a lone electron pair on another atom with strong electronegativity. They are stronger than pd-pd interactions due to the attraction between the hydrogen atom’s positively charged proton and the lone electron pair from the electronegative elements.
The strength of hydrogen bonds varies based on how polarised the bond between the hydrogen atom and the electronegative element is. Bond polarity is in the order of H-F > H-O > H-N. The boiling point of molecules with hydrogen bonding depends on both the bond polarity and the extent of hydrogen bonding. The more hydrogen bonds in a molecule, the higher the boiling point.



Add comment