You probably have heard of the term “alkanes” before but may be wondering what they are. We often encounter Alkanes in our daily lives – natural gas, liquid petroleum gas (LPG), propellants, fuels, and others. Today, we shall discuss what alkanes are and some of the reactions involving alkanes.
What are Alkanes?
As mentioned in Organic Chemistry – An Introduction, alkanes are hydrocarbons with a general formula of CnH2n+2. To be more specific, alkanes are saturated aliphatic hydrocarbons, which means that they only contain carbon and hydrogen atoms. Covalent bonds join adjacent carbon atoms. We have also mentioned that alkanes have a suffix –ane.
Here are some of the simpler alkanes that we commonly encounter.
| n | Name | Molecular formula | Structural formula | Mr | Melting point / °C | Boiling point / °C | Physical state (r.t.p) |
| 1 | Methane | CH4 | CH4 | 16 | -182 | -162 | Gas |
| 2 | Ethane | C2H6 | CH3CH3 | 30 | -183 | -89 | Gas |
| 3 | Propane | C3H8 | CH3CH2CH3 | 44 | -188 | -42 | Gas |
| 4 | Butane | C4H10 | CH3(CH2)2CH3 | 58 | -138 | -0.5 | Gas |
| 5 | Pentane | C5H12 | CH3(CH2)3CH3 | 72 | -130 | 36 | Liquid |
| 6 | Hexane | C6H14 | CH3(CH2)4CH3 | 86 | -95 | 69 | Liquid |
Alkanes with three or more carbon atoms exhibit isomerism. For example, propane has two constitutional isomers (CH3CH2CH2CH3 and CH3CHCH3CH3) whereas butane has three constitutional isomers (CH3CH2CH2CH3, CH3CHCH3CH2CH3, and CH3C(CH3)2CH3).
What are Cycloalkanes?
Cycloalkanes are saturated alicyclic hydrocarbons – this means that they have a ring of carbon atoms. Unlike alkanes, cycloalkanes have a general formula of CnH2n. They are technically different from alkanes and contain the prefix cyclo– to distinguish them from the aliphatic alkanes.
Here are some of the simpler cycloalkanes that we often see.
| n | Name | Molecular formula | Structural formula | Mr | Melting point / °C | Boiling point / °C | Physical state (r.t.p) |
| 3 | Cyclopropane | C3H6 | △ | 42 | -128 | -33 | Gas |
| 4 | Cyclobutane | C4H8 | ◻ | 56 | -91 | 12.5 | Gas |
| 5 | Cyclopentane | C5H10 | ⬠ | 70 | -94 | 49 | Liquid |
| 6 | Cyclohexane | C6H12 | ⬡ | 84 | 6.5 | 81 | Liquid |
Properties of Alkanes
Melting and boiling points
Alkanes’ melting and boiling points differ based on whether it is a straight-chained or branched-chained alkane.
As the number of carbons in the hydrocarbon chain increases, more energy is needed to overcome the stronger instantaneous dipole-induced dipole (id-id) interactions between molecules. As such, the boiling point of the straight-chained alkanes increases. Therefore, the first four alkanes are gases at room temperature, whereas the next thirteen are liquids.
The increasing trend is also seen in the melting points of straight-chained alkanes. However, alkanes with odd-number carbon atoms tend to have a lower melting point than expected.
Branched-chained alkanes often have lower melting points than straight-chained alkanes. This is because they have less surface area of contact between molecules since they are more spherical. As such, there is a decrease in the strength of id-id interactions.
Other physical properties
Alkanes are colourless, non-polar molecules. They are insoluble in polar solvents but are soluble in non-polar solvents. This is due to the energy released between water molecules and alkane molecules being insufficient to overcome the strong hydrogen bonds between water molecules.
On top of that, alkanes are less dense than water. They also become more viscous and less flammable with more carbon atoms.
Preparing Alkanes
Alkanes are produced by reducing alkenes. This is done by heating the alkene with hydrogen gas in the presence of a nickel catalyst. However, we can also reduce alkenes using a platinum or palladium catalyst. This process is also known as hydrogenation.
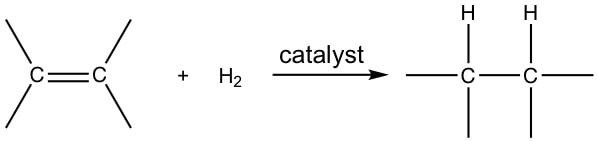
Reactions
Combustion
Alkanes react with oxygen in a combustion reaction, with smaller alkanes igniting more easily. With a limited supply of oxygen, the incomplete combustion of alkanes will produce carbon monoxide (CO) and water (H2O). However, with excess oxygen, the complete combustion of alkanes will produce carbon dioxide (CO2) and water (H2O).
Combustion reactions are exothermic – more energy is produced with more carbon atoms. As alkanes are easily burnt, many of them are used as fuels. One such example is methane (CH4), which is used because it is clean – little soot (carbon) is formed when methane is burnt.
The complete combustion of alkane has the general equation:
CxHy + (x + y/4) O2 → xCO2 + y/2 H2O
Halogenoalkanes
Alkanes form halogenoalkanes when reacted with halogens in a free-radical substitution reaction. This is done under UV light or high temperatures, substituting hydrogen atoms with halogen atoms in the alkanes.
We will be using the chlorination of ethane as an example of a free-radical substitution reaction.
In the initiation step, free radicals Cl• are generated. This occurs when UV light or high temperatures provides energy for the homolytic fission of the Cl–Cl bond.
Cl–Cl → 2Cl•
There is a chain reaction involving the consumption and generation of Cl• radicals in the propagation step. This is due to the consumption of a Cl• radical in one step and the generation of another Cl• radical in the next step.
CH3CH3 + Cl• → CH3ĊH2 + HCl
Cl2 + CH3ĊH2 → CH3CH2Cl + Cl•
In the termination step, free radicals continue to be consumed but are no longer generated. There are many possible termination steps:
Cl• + Cl• → Cl2
CH3ĊH2 + CH3ĊH2 → CH3(CH2)2CH3
CH3ĊH2 + Cl• → CH3CH2Cl
This results in the overall equation of CH3CH3 + Cl2 → CH3CH2Cl + HCl.
Once a single free radical is formed, the chain reaction sequence allows many product molecules to be produced. That said, the reaction is limited to monosubstitution and more than one hydrogen atom in the alkane molecule. To visualise this, let us use the chlorination of methane as an example:
| Monosubstitution | CH4 + Cl2 → CH3Cl + HCl |
| Disubstitution | CH3Cl + Cl2 → CH2Cl2 + HCl |
| Trisubstitution | CH2Cl + Cl2 → CHCl3 + HCl |
| Tetrasubstitution | CHCl3+ Cl2 → CCl4 + HCl |
Reactivity and Substitution
As we know, the reactivity of halogens decreases down the group. This makes fluorine and iodine unsuitable for free-radical substitution – fluorine is too reactive, whereas iodine is too unreactive. In addition, down the group, as halogens have a larger atomic radius, the energy released when the H–X bond is formed is less than that needed to break the C–H bonds in an alkane. This makes the first propagation step more endothermic, and thus it becomes increasingly harder to substitute the halogen.
When halogens substitute hydrogen atoms on different carbon atoms, other products are formed. However, this is more commonly seen in higher alkanes.
Occurrence of Alkanes
When I first learnt about this, I was a little surprised – we can also find alkanes in the atmospheres of other planets! Jupiter, Saturn, Uranus, and Neptune have a small portion of methane and ethane in their atmospheres. Saturn’s largest moon, Titan, often encounters rains made of liquid methane.
A small amount of gaseous alkanes can be found in our atmosphere on Earth – namely methane. This is partially due to air pollution, which is formed when we burn fossil fuels (alkanes). However, hydrocarbons can be found deep within Earth’s surface from the fossils of dead marine life over millions of years ago.



Add comment