Most of us know what atoms are – they are so small that almost none of us have seen them with our own eyes. However, with the advancement of technology and science, we can now visualise and understand them and their functions. Today, we will discuss the different parts of an atom, and the different types of atomic structures involved.
Atoms
Atoms are the smallest particles of an element with the same chemical properties as that element. They are made up of sub-atomic particles – protons, neutrons, and electrons. We will discuss them in detail in a while.
The relative atomic mass, Ar, of an element is the ratio of the average mass of an atom of the element to 1/12 the mass of a 12C isotope. An atom’s mass is concentrated on its nucleus as its protons and neutrons have equal mass, whereas its electrons have negligible mass.
Every element is comprised of atoms, and the atoms of the same element are similar to each other. Elements are pure substances that cannot be further split into simpler substances chemically or electrically. Two or more types of atoms form compounds, which are pure substances comprised of two or more elements chemically combined in a fixed ratio.
Sub-atomic Particles
As mentioned above, there are three types of sub-atomic particles. Protons and neutrons are closely packed in the nucleus of the atom, whereas electrons are distributed around the atom. Together, protons and neutrons are known as nucleons. As electrons are distributed around the atom, they occupy most of the atom’s volume but have a negligible percentage of the atomic mass.
Here is a summary of the different sub-atomic particles.
| Protons | Neutrons | Electrons | |
| Charge relative to proton | 1 | 0 | -1 |
| Mass relative to proton | 1 | 1 | 1/1840 |
| Electric charge / C | +1.062×10-19 | 0 | -1.062×10-19 |
| Mass / kg | 1.67×10-27 | 1.67×10-27 | 9.11×10-31 |
Sub-atomic particles in an electric field
When a beam with sub-atomic particles passes through an electric field, the different sub-atomic particles will deflect differently. Both protons and electrons will be deflected, whereas neutrons continue to move in a straight path perpendicular to the electric field. However, the angle of deflection of the protons and electrons are different. This depends on the relative charge and mass of the particles.
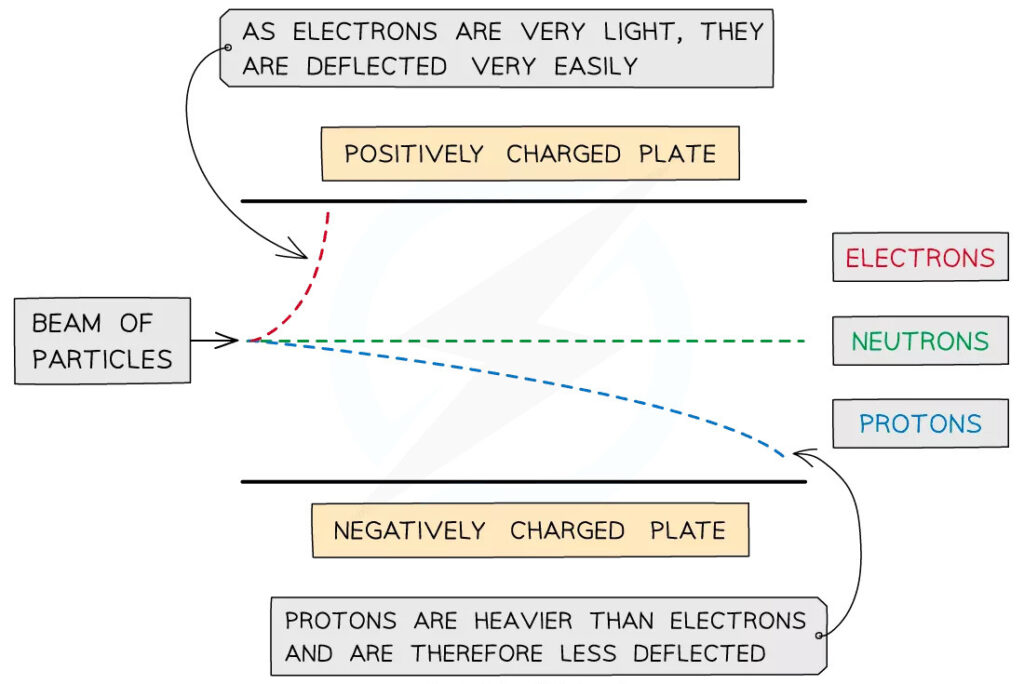
We can calculate the angle of deflection with the formula:
Angle ∝ charge ÷ mass
Since electrons have negligible mass, electrons have a greater angle of deflection compared to protons. Particles with higher charges or lower masses will have a greater angle of deflection.
Isotopes
Isotopes are atoms of the same element with the same number of protons and electrons but a different number of electrons. They have similar chemical properties but different physical properties.
Ions
Ions are charged particles that are formed when an atom or a group of atoms gain or lose electrons. Ions are formed to achieve noble gas configuration with a fully filled valence electron shell.
Group I to Group III elements, together with hydrogen, form positive cations when they lose electrons. On the other hand, Group XV to Group XVII elements form negative cations when they gain electrons. This occurs as it is easier for the respective elements to achieve noble gas configuration when they lose or gain electrons, respectively.
However, Group XIV elements seldom form ions as it is difficult to gain or lose the four electrons in their valence electron shells. Similarly, Group 0 elements (noble gases) already have a fully filled valence electron shell and thus do not form ions.
Electronic Structures
Electron shells
In an atom, electrons are arranged in shells with a principal quantum number. The principal quantum number is arranged such that shell 1 is the closest to the nucleus and so on. As such, the lower the principal quantum number, the closer the shell is to the nucleus.
Negatively-charged electrons in shells with lower principal quantum numbers have a greater electrostatic attraction with the positively-charged protons in the nucleus. This makes shells filled in the order of increasing energy. As such, these electrons are more stable compared to electrons in shells further away from the nucleus. Shells contain 2n2 electrons, where n represents the principal quantum number.
Subshells
Shells have subshells “s”, “p”, “d”, “f”, and “g”. However, subshell g is seldom used. The number of subshells in a shell is correspondent to the principle quantum number. To explain this further:
| n | Subshells | Subshell types | Subshell names |
| 1 | 1 | s | 1s |
| 2 | 2 | s, p | 2s, 2p |
| 3 | 3 | s, p, d | 3s, 3p, 3d |
| 4 | 4 | s, p, d, f | 4s, 4p, 4d, 4f |
Electrons within a subshell are degenerate – they have the same energy level.
Orbitals
Orbitals are regions of space where locating electrons is highly probably. The number of orbitals per subshell depends on its type – each orbital can only contain two electrons. To simplify this:
| Subshell type | Number of orbitals | Maximum electrons per subshell |
| s | 1 | 1×2=2 |
| p | 3 | 3×2=6 |
| d | 5 | 5×2=10 |
| f | 7 | 7×2=14 |
On top of this, different orbitals have different shapes. Here is an overview of all the different shapes of the various types of subshells.
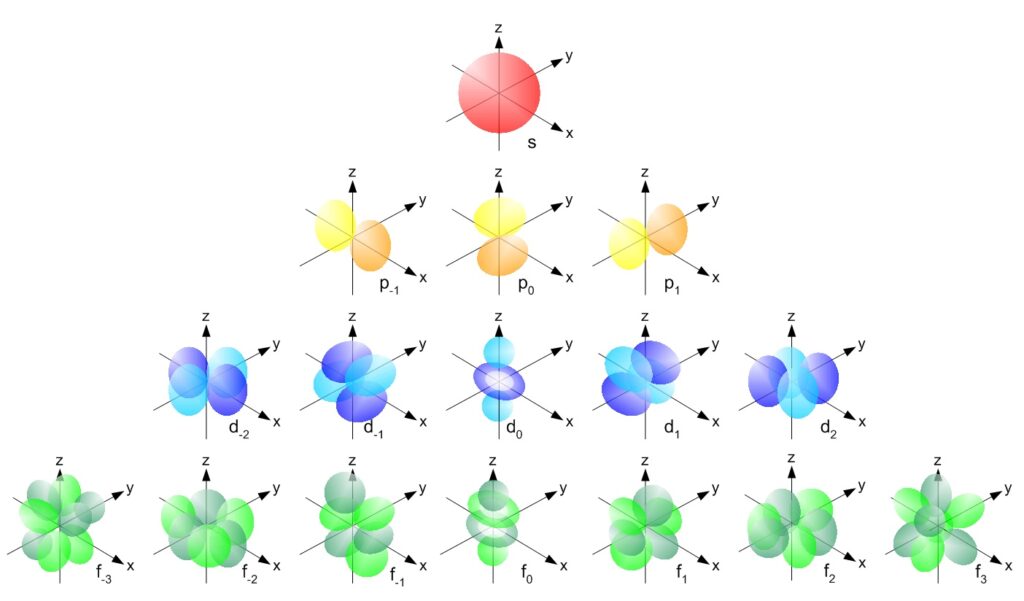
s-orbitals
S-orbitals are spherical in shape. All s-orbitals have the same shape but have different sizes based on which shell they are from.
p-orbitals
P-orbitals are dumbbell in shape. There are three types of p-orbitals – px, py, and pz with different configurations and orientations. Similar to s-orbitals, they have the same shape but have different sizes based on which shell they are from.
d-orbitals
Of five d-orbitals that exist, four of them have a similar four-leaved clover shape. Similarly, the orbitals have different sizes based on which shell they are from.
f-orbitals
F-orbitals have complex shapes. They are often omitted from textbooks and are seldom discussed.
Periodic Table
Now that we have discussed the different types of orbitals, we can talk about how they are portrayed in the periodic table. As there are four types of orbitals, the periodic table can be represented in four blocks:
s-block – Group I and Group II
p-block – Group XIII to Group 0
d-block – Group III to Group XII (transition metals)
f-block – Lanthanides and Actinides
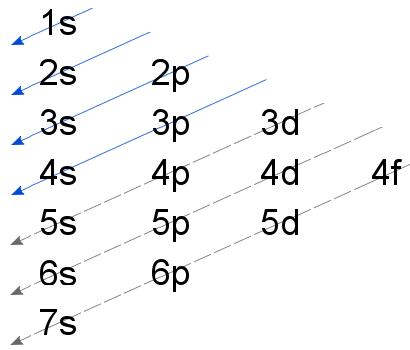
Aufbau Principle
The Aufbau principle states that electrons always occupy lower energy orbitals before occupying higher energy orbitals. The relative energies of orbitals, and the order in which electrons fill the orbitals, are shown below.
Conclusion
Now that you have understood the concept of atomic structure, you should be able to visualise the elements in the periodic table. As this is the fundamental of all Chemistry, it is important for you to understand this thoroughly.



Add comment